SMA Specialty Medical Lab – Molecular Microbiology Services
The Clinical Microbiology Laboratory at SMA offers the following panels:

- Urinary Tract Infection (UTI) Panel
- Wound Infection Panel
- Vaginal Panel
- STD Panel
- GI Bacterial Panel
- Small Respiratory Panel
- Respiratory Panel
- Covid-19 PCR Testing
- Nail Fungi PCR Panel
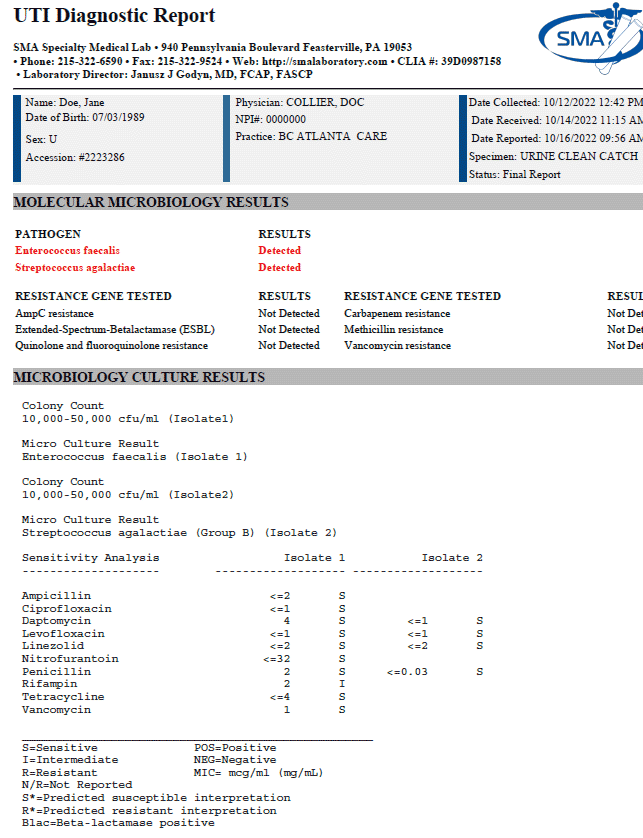
Urinary Tract Infection (UTI) Panel
Clinical Significance:
The gold standard for UTI diagnosis is urine culture, which takes 2-3 days for organism identification and susceptibility. Molecular techniques offer a means of rapid identification of microorganisms and resistance genes for rapid diagnosis of complicated, recurrent UTIs and high risk patients.
Benefits include:
- Accurate and Rapid results
- More Answers for Polymicrobial UTIs
- Informed Treatment Decisions Based on Resistance Genes Detected
- Superior Sensitivity and Specificity for microbial detection
- TEST ORDERING CODE: 6300
- METHODOLOGY: PCR
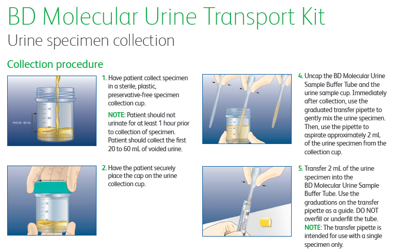
- SPECIMEN REQUIREMENTS: CLEAN CATCH URINE IN GRAY TOP TUBE OR STERILE URINE CONTAINER; BRIEF SWAB
- MINIMUM VOLUME: 3 mls
- TEMPERATURE STABILITY: Room temp (15-25°C) for up to 24 hrs (urine cup), up to 48 hrs (gray top tube); 5 days if refrigerated (2-8°C); brief swab-48 hrs
- TURNAROUND TIME: <24 hrs from receipt Tues-Sat
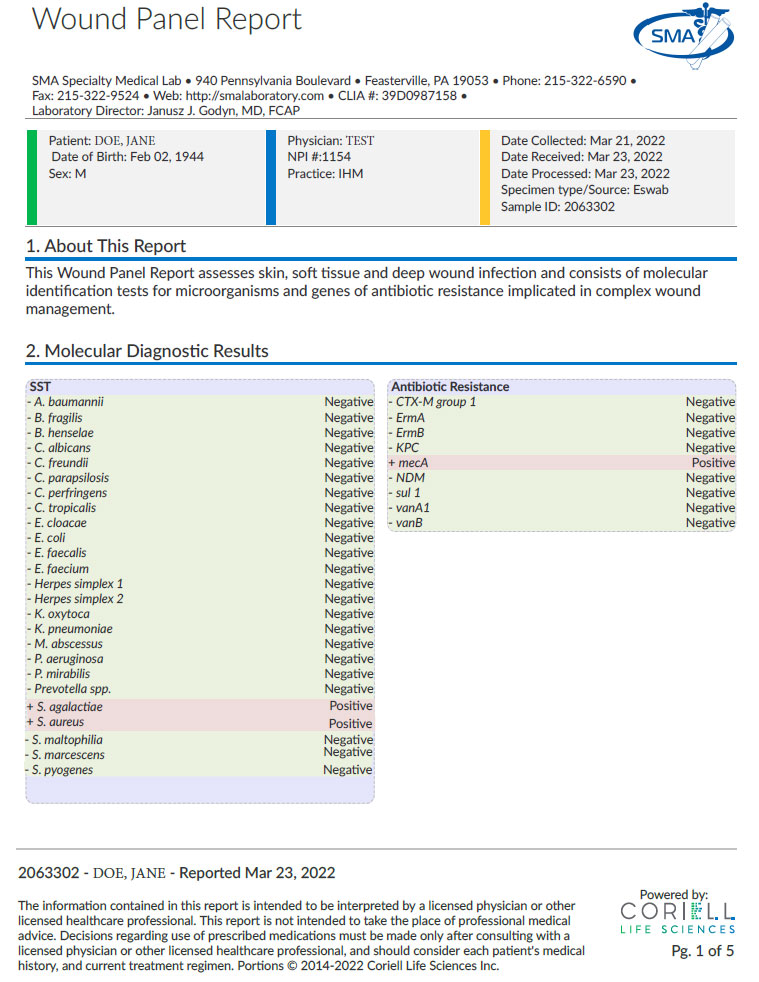
Wound Infection Panel
Clinical Significance:
The gold standard for skin and soft tissue infection is wound culture, which takes a minimum of 3-5 days for aerobic and anaerobic organism identification and susceptibility. Molecular techniques offer a means of rapid identification of microorganisms and resistance genes for rapid diagnosis of complicated wound infections.
Benefits include:
- Accurate and Rapid results including anaerobic organism identification
- More Answers for Polymicrobial Wound Infections
- Informed Treatment Decisions Based on Resistance Genes Detected
- Superior Sensitivity and Specificity for microbial detection
- TEST ORDERING CODE: 6301
- METHODOLOGY: PCR
- SPECIMEN REQUIREMENTS: Eswab Collection and Transport Tube for Aerobic and Anaerobic Organisms (BD or Copan)
- TEMPERATURE STABILITY: Room temp (15-25°C) for up to 48 hrs; refrigeration DOES NOT extend the stability of the specimen.
- TURNAROUND TIME: <48 hrs from receipt Tues-Sat
*See the molecular brochure for more test details
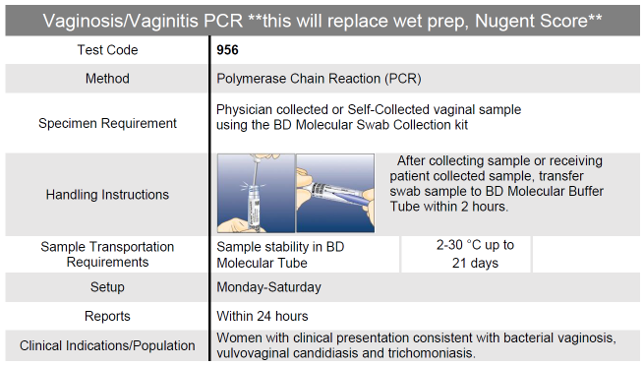
Vaginal Panel- Vaginosis/Vaginitis
Clinical Significance: Vaginitis is one of the 25 most common medical reasons for consulting a physician in the U.S., resulting in 5 to 10 million office visits per year.2 Recent data indicates that traditional diagnostics leave up to 40% of women with vaginitis undiagnosed after an initial clinician visit.3
Benefits include:
- Accurate and Rapid results
- Direct detection of Bacterial Vaginosis using a superior microbiome bases algorithm
- Simulataneous detection of Trichomonas vaginalis, Candida species, Candida krusei and Candida glabrata
- Clinician collected or Patient collected
- Superior Sensitivity and Specificity
Vaginal Panel PCR
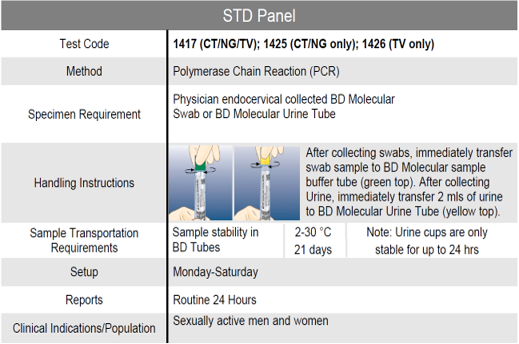
STD Panel
Clinical Significance:
The World Health Organization estimates that 130.9 million new cases of infection due to Chlamydia trachomatis, 78.3 million new cases of Neisseria gonorrhoeae, and 142.6 million new trichomoniasis cases are diagnosed each year.1 Undiagnosed infection can result in several acute and chronic conditions for both men and women.
Benefits include:
- Rapid same day detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis
- Test can be performed from both urine samples and vaginal swabs.
- One vaginal swab can be used for the STD panel and the Vaginal panel.
Bacterial GI Panel
Among reported outbreaks of bacterial diarrhea, four of the major pathogens isolated are Campylobacter spp., Salmonella spp., Shigella spp., and Shiga toxin producing E. coli. Of these, non-typhoidal Salmonella species are the leading cause of hospitalization and death among food borne bacteria.
| GI BACTERIAL PANEL | |
| Test Code | 958 |
| Method | Polymerase Chain Reaction (PCR) |
| Specimen Requirements | Unpreserved Soft to Diarrheal Stool or Cary-Blair preserved stool |
| Included in this test | Salmonella sp Campylobacter spp. Shigella spp. (including enteroinvasive Escherichia coli (EIEC) |
| Sample Transportation Requirements |
5 Days if Refrigerated 24 hours if Room Temp |
| Setup | Monday-Saturday |
| Reports | Routine 24 hours |
| Clinical Indications/Population | Patients experiencing persistent or chronic diarrhea, bloody stools/persistent abdominal pain |
Respiratory Panels
SMA currently offers the following 3 PCR based respiratory test:
| Test Code: C19 | Covid 19, PCR |
| Performing Lab: | SMA Specialty Medical Lab |
| TEST INCLUDES | Qualitative Detection of Sars-CoV-2 |
| Reference Ranges: | Detected |
| Not Detected | |
| Volume | 2-3 mls of Transport Media (Saline, VTM, MTM and Liquid Aimes) |
| Specimen | Nasopharyngeal swab |
| container | Nasopharyngeal swab in Transport Media |
| Collection | Insert flexible minitip swab through the nares parallel to the palate (not upwards) until resistance is encountered or the distance isequivalent to that from the ear to the nostril of the patient, indicating contact with the nasopharynx. Gently rotate the swab 2-3 times and slowly remove. Place swabs immediately into sterile tubes containing 2-3 mL of viral transport media. Be sure to closes tube tightly to prevent leakage in transport. Label tube with patient name and birth date. |
| Transport Temperature: | Store swabs in refrigeration at 2-8˚C for up to 72 hours and transport specimen on ice. Samples collected more than 72 hours ago MUST be stored at -70˚C and transported with dry ice. |
| Specimen Stability: | Room temperature for 24 hours; Refrigerated for 72 hours; -70˚C for greater than 72 hours. |
| Causes for Rejection | • Swabs with calcium alginate or cotton tips with wooden shafts used for collection. • Refrigerated samples are greater than 72 hours old. • Samples received/ transported at room temperature (no ice/no dry ice). • Broken or leaking transport tubes. • Improper collection tube. |
| Methodology: | Real Time PCR |
| Setup Days: | Monday – Sunday |
| Setup Times: | Day Shift |
| Turnaround: | 24 hours from Reciept |
| CPT Code(s): | U0003 |
| (The CPT codes provided are based on AMA guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. Please direct any questions regarding coding to the payer being billed.) | |
| use | Detection of SARS-CoV-2 virus |
| Test Code: 976 | 3 Target Respiratory Panel, PCR |
| Performing Lab: | SMA Specialty Medical Lab |
| TEST INCLUDES | Qualitative Detection of Influenza A, Influenza B, and RSV |
| Reference Ranges: | Detected |
| Not Detected | |
| Volume | 2-3 mls of Transport Media (Saline, VTM, MTM and Liquid Aimes) |
| Specimen | Nasopharyngeal swab |
| container | Nasopharyngeal swab in Transport Media |
| Collection | Insert flexible minitip swab through the nares parallel to the palate (not upwards) until resistance is encountered or the distance isequivalent to that from the ear to the nostril of the patient, indicating contact with the nasopharynx. Gently rotate the swab 2-3 times and slowly remove. Place swabs immediately into sterile tubes containing 2-3 mL of viral transport media. Be sure to closes tube tightly to prevent leakage in transport. Label tube with patient name and birth date. |
| Transport Temperature: | Store swabs in refrigeration at 2-8˚C for up to 72 hours and transport specimen on ice. Samples collected more than 72 hours ago MUST be stored at -70˚C and transported with dry ice. |
| Specimen Stability: | Room temperature for 24 hours; Refrigerated for 72 hours; -70˚C for greater than 72 hours. |
| Causes for Rejection | • Swabs with calcium alginate or cotton tips with wooden shafts used for collection. • Refrigerated samples are greater than 72 hours old. • Samples received/ transported at room temperature (no ice/no dry ice). • Broken or leaking transport tubes. • Improper collection tube. |
| Methodology: | Real Time PCR |
| Setup Days: | Monday – Sunday |
| Setup Times: | Day Shift |
| Turnaround: | 24 hours from Reciept |
| CPT Code(s): | 87631 |
| (The CPT codes provided are based on AMA guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. Please direct any questions regarding coding to the payer being billed.) | |
| use | Detection of Flu A, Flu B, and RSV viruses |
| Test Code: 966 | Respiratory Profile, PCR |
| Performing Lab: | SMA Specialty Medical Lab |
| Clinical Significance: | Qualitative detection and identification of multiple respiratory viral and bacterial nucleic acids in nasopharyngeal swabs obtained from individuals suspected of respiratory tract infection |
| TEST INCLUDES | Respiratory Syncytial Virus A (RSV A) Respiratory Syncytial Virus B (RSV B) Influenza A Influenza A_H1N1_ pdm09 Influenza A_H1 Influenza A_H3 Influenza B Parainfluenza virus 1 Parainfluenza virus 2 Parainfluenza virus 3 Parainfluenza virus 4 Human Enterovirus Adenovirus Metapneumovirus Coronavirus 229E Coronavirus NL63 Coronavirus OC43 Human Bocavirus Human Rhinovirus Streptococcus pneumoniae Legionella pneumophila Haemophilus influenzae Mycoplasma pneumoniae Chlamydia pneumoniae Bordatella pertussis Bordatella parapertussis |
| Reference Ranges: | Detected |
| Not Detected | |
| Minimum Volume | 2-3 ml |
| Preferred Specimen | Nasopharyngeal swab |
| Specimen Container | Nasopharyngeal swab in Viral Transport Media |
| Collection | Insert flexible minitip swab through the nares parallel to the palate (not upwards) until resistance is encountered or the distance isequivalent to that from the ear to the nostril of the patient, indicating contact with the nasopharynx. Gently rotate the swab 2-3 times and slowly remove. Place swabs immediately into sterile tubes containing 2-3 mL of viral transport media. Be sure to closes tube tightly to prevent leakage in transport. Label tube with patient name and birth date. |
| Transport Temperature: | Store swabs in refrigeration at 2-8˚C for up to 72 hours and transport specimen on ice. Samples collected more than 72 hours ago MUST be stored at -70˚C and transported with dry ice. |
| Specimen Stability: | ROOM TEMPERATURE: 24 HOURS REFRIGERATED: 3 DAYS FROZEN AT -70°C 30 DAYS |
| Causes for Rejection | • Swabs received in saline, PBS, liquid aimes, or any other media other than viral transport media. • Swabs with calcium alginate or cotton tips with wooden shafts used for collection. • Refrigerated samples that are greater than 72 hours old. • Samples received/ transported at room temperature for more than 24 hours (no ice/no dry ice). • Broken or leaking transport tubes. • Improper collection tube. |
| Methodology: | Polymerase chain reaction (PCR) |
| Setup Days: | 1 |
| Setup Times: | First Shift |
| Turnaround: | 24 HOURS (SPECIMENS RECIEVED AFTER 12 P.M. ARE CONSIDERED RECIEVED ON NEXT BUSINESS DAY AND WILL BE PROCESSED ACCORDINGLY) |
| CPT Code(s): | 87631, 87798 x 4, 87541, 87581, 87486 |
| (The CPT codes provided are based on AMA guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. Please direct any questions regarding coding to the payer being billed.) | |
| Instrument: | BIORAD CFX96 TOUCH REAL-TIME PCR SYSTEM |
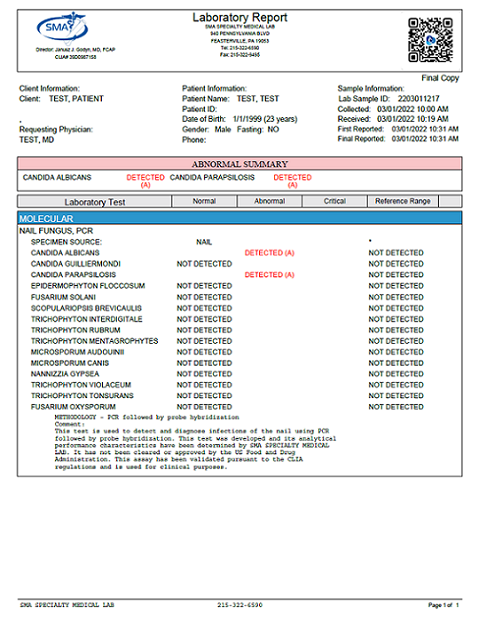
Nail Fungal Panel, PCR
| Test Code: 977 | Nail Fungal Panel, PCR |
| Performing Lab: | SMA Specialty Medical Lab |
| Clinical Significance: | Qualitative detection and identification of multiple nail fungal pathogens |
| Reference Ranges: | Not Detected |
| TEST COMPONENTS | Candida albicans |
| Candida guilliermondi | |
| Candida parapsilosis | |
| Epidermophyton floccosum | |
| Fusarium solani | |
| Scopulariopsis brevicaulis | |
| Trichophyton interdigitale | |
| Trichophyton rubrum | |
| Trichophyton mentagrophytes | |
| Microsporum audouinii | |
| Microsporum canis | |
| Nanizzia gypsea | |
| Trichophyton violaceum | |
| Trichophyton tonsurans | |
| Fusarium oxysporum | |
| Preferred Specimen: | Nail Trimmings |
| Alternate Specimens: | No |
| Minimum Volume: | 1 Nail Clipping |
| Specimen Container: | Sterile Container |
| Transport Temperature: | Room Temperature |
| Specimen Stability: | Room Temperature for 1 week |
| Refrigerated for 4 weeks | |
| Methodology: | Polymerase chain reaction (PCR) |
| Setup Days: | Monday-Saturday |
| Setup Times: | FIRST SHIFT |
| Turnaround: | 48 hours from receipt |
| CPT Code(s): | 87481, 87798 |
| (The CPT codes provided are based on AMA guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. Please direct any questions regarding coding to the payer being billed.) | |
| INSTRUMENT | CFX96 Touch Real-Time PCR System |